Introduction
About 30 % of the proteins encoded by the human genome code for transmembrane proteins, highlighting their importance for human physiology. Our lab focuses on a family of transmembrane proteins known as ion channels. These molecular switches regulate the flux of ions across the otherwise ion-impermeable hydrophobic core of biological membranes. Ion channels play crucial roles in numerous physiological processes, such as neuronal and cardiac excitability. Genetic or acquired dysfunctions in ion channels are the known cause of pain, epilepsy, and cardiac arrhythmias, making them front-line therapeutic targets.
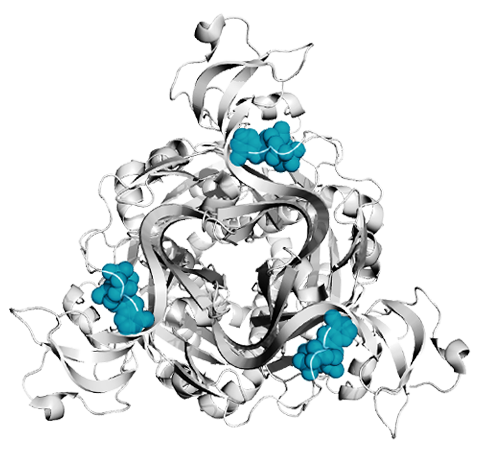
Left: Top down view of a P2X receptor with ATP shown in blue

Trimeric ion channels
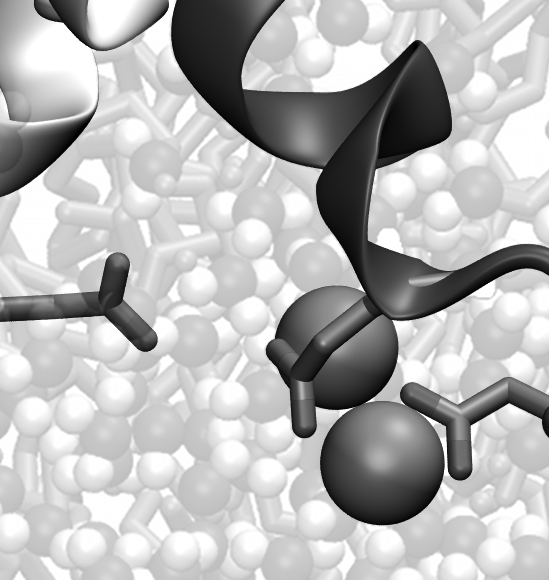
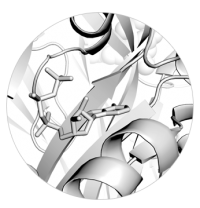
A major focus of our lab are acid-sensing ion channels (ASICs) and P2X receptors. They are trimeric ligand-gated ion channels that open cation-selective pores in response to binding of extracellular protons and ATP, respectively. They play important roles in synaptic transmission throughout the body and their dysfunction has been implicated in numerous diseases, such as hearing loss, pain and stroke (Heusser & Pless, TiPS, 2022; Illes et al., Br J Pharm, 2020). We are interested in all molecular aspects of these ion channels, including basic mechanism of activation, origin of their ion selectivity, pharmacology, as well as functional modulation by protein-protein interactions.
Left: Sodium ions binding to the ASIC selectivity filter

NALCN channel complex
Another key area of interest is the sodium leak channel NALCN. This ion channel is required for survival in mammals and gain- or loss-of-function mutations can result in devastating diseases in humans. We have recently shown that NALCN is only functional when it forms a complex with three other proteins, FAM155A, UNC79 and UNC80 (Chua et al, Science Advances, 2020). This breakthrough enabled the first detailed functional characterisation of NALCN and led our collaborators to obtain a structure of the entire NALCN channelsome, along with mechanistic and pharmacological insight (Kschonsak et al, Nature, 2020 and 2022). We continue to study the function and pharmacology, as well as disease-causing mutations of the NALCN channelsome.
Left: Close up view of ATP bound to a P2X receptor

last section
Recent progress in structure determination of trimeric ion channels and the sodium leak channel (Kschonsak et al., Nature, 2022; Yoder & Gouaux, eLife, 2020; McCarthy et al., Cell, 2019) provides an excellent framework to shed new light on these fascinating macromolecules. In particular, our lab employs a combination of electrophysiology, chemical biology, protein engineering and fluorescence spectroscopy to address crucial questions regarding the molecular function and pharmacology of these proteins:
- ElectrophysiologyWhen ions flow across a membrane they generate electrical currents. Although minute, they can be measured with great precision, down to the level of ions flowing through a single ion channel. In our lab we employ a variety of electrophysiological recording techniques, e.g. two-electrode voltage-clamp and patch-clamp methodologies, including single molecule and high throughput recordinds (Lynagh et al., eLife, 2017; Braun et al., PLoS Biol, 2021).
- Protein engineeringEmerging chemical biology techniques allow the site-directed incorporation of ncAAs or post-translational modifications via either non-sense suppression approaches (Braun et al, J Physiol, 2020) or protein semi-synthesis (Khoo & Galleano et al., Nat Comm, 2020, Galleano & Harms, PNAS, 2021). These modifications offer the ability to either incorporate subtle derivatives of naturally occurring amino acids or to confer entirely new properties to a given protein.
- FluorometryPatch-clamp fluorometry (PCF) and its cousin voltage-clamp fluorometry (VCF) allow labeling an ion channel with an organic dye. When the protein changes its conformation, the dye reports a change in environment by changing its brightness (Borg & Braun et al, PNAS, 2020; Heusser & Borg et al, eLife, 2022). We recently increased the sensitivity of this approach, allowing to track protein conformational changes independent of ionic currents with greater temporal resolution (Wulf & Pless, Cell Rep, 2018).